6.12.2 Direct GWPs
The CO2 response function used in this report is the same as that in WMO (l999)
and the SAR and is based on the “Bern” carbon cycle model (see Siegenthaler
and Joos, 1992; Joos et al., l996) run for a constant future mixing ratio of
CO2 over a period of 500 years. The Bern carbon cycle model was compared to
others in IPCC (l994), where it was shown that different models gave a range
of as much as 20% in the CO2 response, with the greatest differences occurring
over time-scales greater than 20 years.
The radiative efficiency per kilogram of CO2 has been updated compared to previous
IPCC assessments (IPCC, 1994; SAR). Here we employ the approach discussed in
WMO (l999) using the simplified formula presented in Table
6.2. We assume a background CO2 mixing ratio of 364 ppmv, close to the present
day value (WMO (1995) used 354 ppmv). For this assumption, this expression agrees
well with the adjusted total-sky radiative forcing calculations of Myhre and
Stordal (1997); see also Myhre et al. (1998b). The revised forcing is about
12% lower than that in the SAR. For a small perturbation in CO2 from 364 ppmv,
the radiative efficiency is 0.01548 Wm-2/ppmv. This value is used in the GWP
calculations presented here. We emphasise that it applies only to GWP calculations
and cannot be used to obtain the total radiative forcing for this key gas since
pre-industrial times, due to time-dependent changes in mixing ratio as noted
above. Because of this change in the CO2 forcing per mass, the CO2 AGWPs are
0.207, 0.696, and 2.241 Wm-2/yr /ppmv for 20, 100, and 500 year time horizons,
respectively. These are smaller than the values used in the SAR by 13%. AGWPs
for any gas can be obtained from the GWP values given in the tables presented
here by multiplying by these numbers.
The decreases in the CO2 AGWPs will lead to proportionately larger GWPs for
other gases compared to previous IPCC assessments, in the absence of other changes.
In Tables 6.7 and 6.8, GWPs
(on a mass basis) for 93 gases are tabulated for time horizons of 20, 100, and
500 years. The list includes CH4, N2O, CFCs, HCFCs, HFCs, hydrochlorocarbons,
bromocarbons, iodocarbons, fully fluorinated species, fluoroalcohols, and fluoroethers.
The radiative efficiencies per kilogram were derived from the values given per
ppbv in Section 6.3. Several of these have been updated
since the SAR, most notably that of CFC-11. Since the radiative forcings of
the halocarbon replacement gases are scaled relative to CFC-11 in GWP calculations,
the GWPs of those gases are also affected by this change. As discussed further
in Section 6.3, the change in radiative forcing for CFC-11
reflects new studies (Pinnock et al., 1995; Christidis et al., l997; Hansen
et al., l997a; Myhre and Stordal, 1997; Good et al., 1998) suggesting that the
radiative forcing of this gas is about 0.25 Wm-2 ppbv-1, an increase
of about 14% compared to the value adopted in earlier IPCC reports, which was
based on the study of Hansen et al. (l988).
| Table 6.7: Direct Global Warming Potentials (mass
basis) relative to carbon dioxide (for gases for which the lifetimes have
been adequately characterised). |
 |
|
Gas
|
Radiative efficiency (Wm-2 ppb-1)
(from (b) unless indicated)
|
Lifetime (years) (from Chapter
4 unless indicated)
|
Global Warming Potential
Time horizon
 |
| |
|
| |
|
| |
|
20 years
|
100 years
|
500 years
|
 |
| Carbon dioxide |
CO2 |
See Section 6.12.2 |
See Section 6.12.2 |
1
|
1
|
1
|
| Methane |
CH4 |
3.7x10-4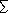 |
12.0* |
62
|
23
|
7
|
| Nitrous oxide |
N2O |
3.1x10-3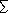 |
114* |
275
|
296
|
156
|
| Chlorofluorocarbons |
| CFC-11 |
CCl3F |
0.25 |
45 |
6300
|
4600
|
1600
|
| CFC-12 |
CCl2F2 |
0.32 |
100 |
10200
|
10600
|
5200
|
| CFC-13 |
CClF3 |
0.25 |
640 (c) |
10000
|
14000
|
16300
|
| CFC-113 |
CCl2FCClF2 |
0.30 |
85 |
6100
|
6000
|
2700
|
| CFC-114 |
CClF2CClF2 |
0.31 |
300 |
7500
|
9800
|
8700
|
| CFC-115 |
CF3CClF2 |
0.18† |
1700 |
4900
|
7200
|
9900
|
| Hydrochlorofluorocarbons |
| HCFC-21 |
CHCl2F |
0.17 |
2.0 (d) |
700
|
210
|
65
|
| HCFC-22 |
CHClF2 |
0.20§ |
11.9 |
4800
|
1700
|
540
|
| HCFC-123 |
CF3CHCl2 |
0.20 |
1.4 (a) |
390
|
120
|
36
|
| HCFC-124 |
CF3CHClF |
0.22 |
6.1 (a) |
2000
|
620
|
190
|
| HCFC-141b |
CH3CCl2F |
0.14 |
9.3 |
2100
|
700
|
220
|
| HCFC-142b |
CH3CClF2 |
0.20 |
19 |
5200
|
2400
|
740
|
| HCFC-225ca |
CF3CF2CHCl2 |
0.27 |
2.1 (a) |
590
|
180
|
55
|
| HCFC-225cb |
CClF2CF2CHClF |
0.32 |
6.2 (a) |
2000
|
620
|
190
|
| Hydrofluorocarbons |
| HFC-23 |
CHF3 |
0.16§ |
260 |
9400
|
12000
|
10000
|
| HFC-32 |
CH2F2 |
0.09§ |
5.0 |
1800
|
550
|
170
|
| HFC-41 |
CH3F |
0.02 |
2.6 |
330
|
97
|
30
|
| HFC-125 |
CHF2CF3 |
0.23§ |
29 |
5900
|
3400
|
1100
|
| HFC-134 |
CHF2CHF2 |
0.18 |
9.6 |
3200
|
1100
|
330
|
| HFC-134a |
CH2FCF3 |
0.15§ |
13.8 |
3300
|
1300
|
400
|
| HFC-143 |
CHF2CH2F |
0.13 |
3.4 |
1100
|
330
|
100
|
| HFC-143a |
CF3CH3 |
0.13§ |
52 |
5500
|
4300
|
1600
|
| HFC-152 |
CH2FCH2F |
0.09 |
0.5 |
140
|
43
|
13
|
| HFC-152a |
CH3CHF2 |
0.09§ |
1.4 |
410
|
120
|
37
|
| HFC-161 |
CH3CH2F |
0.03 |
0.3 |
40
|
12
|
4
|
| HFC-227ea |
CF3CHFCF3 |
0.30 |
33.0 |
5600
|
3500
|
1100
|
| HFC-236cb |
CH2FCF2CF3 |
0.23 |
13.2 |
3300
|
1300
|
390
|
| HFC-236ea |
CHF2CHFCF3 |
0.30 |
10.0 |
3600
|
1200
|
390
|
| HFC-236fa |
CF3CH2CF3 |
0.28 |
220 |
7500
|
9400
|
7100
|
| HFC-245ca |
CH2FCF2CHF2 |
0.23 |
5.9 |
2100
|
640
|
200
|
| HFC-245fa |
CHF2CH2CF3 |
0.28& |
7.2 |
3000
|
950
|
300
|
| HFC-365mfc |
CF3CH2CF2CH3 |
0.21 (k) |
9.9 |
2600
|
890
|
280
|
| HFC-43-10mee |
CF3CHFCHFCF2CF3 |
0.40 |
15 |
3700
|
1500
|
470
|
| Chlorocarbons |
| CH3CCl3 |
|
0.06 |
4.8 |
450
|
140
|
42
|
| CCl4 |
|
0.13†† |
35 |
2700
|
1800
|
580
|
| CHCl3 |
|
0.11§ |
0.51 (a) |
100
|
30
|
9
|
| CH3Cl |
|
0.01 |
1.3 (b) |
55
|
16
|
5
|
| CH2Cl2 |
|
0.03 |
0.46 (a) |
35
|
10
|
3
|
| Bromocarbons |
| CH3Br |
|
0.01 |
0.7 (b) |
16
|
5
|
1
|
| CH2Br2 |
|
0.01 |
0.41 (i) |
5
|
1
|
<<1
|
| CHBrF2 |
|
0.14 |
7.0 (i) |
1500
|
470
|
150
|
| Halon-1211 |
CBrClF2 |
0.30 |
11 |
3600
|
1300
|
390
|
| Halon-1301 |
CBrF3 |
0.32 |
65 |
7900
|
6900
|
2700
|
| Iodocarbons |
| CF3I |
|
0.23 |
0.005 (a) |
1
|
1
|
<<1
|
| Fully fluorinated species |
| SF6 |
|
0.52 |
3200 |
15100
|
22200
|
32400
|
| CF4 |
|
0.08 |
50000 |
3900
|
5700
|
8900
|
| C2F6 |
|
0.26§ |
10000 |
8000
|
11900
|
18000
|
| C3F8 |
|
0.26 |
2600 |
5900
|
8600
|
12400
|
| C4F10 |
|
0.33 |
2600 |
5900
|
8600
|
12400
|
| c-C4F8 |
|
0.32§ |
3200 |
6800
|
10000
|
14500
|
| C5F12 |
|
0.41 |
4100 |
6000
|
8900
|
13200
|
| C6F14 |
|
0.49 |
3200 |
6100
|
9000
|
13200
|
| Ethers and Halogenated Ethers |
| CH3OCH3 |
|
0.02 |
0.015 (e) |
1
|
1
|
<<1
|
| (CF3)2CFOCH3 |
|
0.31 |
3.4 (l) |
1100
|
330
|
100
|
| (CF3)CH2OH |
|
0.18 |
0.5 (m) |
190
|
57
|
18
|
| CF3CF2CH2OH |
|
0.24 |
0.4 (m) |
140
|
40
|
13
|
| (CF3)2CHOH |
|
0.28 |
1.8 (m) |
640
|
190
|
59
|
| HFE-125 |
CF3OCHF2 |
0.44 |
150 |
12900
|
14900
|
9200
|
| HFE-134 |
CHF2OCHF2 |
0.45 |
26.2 |
10500
|
6100
|
2000
|
| HFE-143a |
CH3OCF3 |
0.27 |
4.4 |
2500
|
750
|
230
|
| HCFE-235da2 |
CF3CHClOCHF2 |
0.38 |
2.6 (i) |
1100
|
340
|
110
|
| HFE-245cb2 |
CF3CF2OCH3 |
0.32 |
4.3 (l) |
1900
|
580
|
180
|
| HFE-245fa2 |
CF3CH2OCHF2 |
0.31 |
4.4 (i) |
1900
|
570
|
180
|
| HFE-254cb2 |
CHF2CF2OCH3 |
0.28 |
0.22 (h) |
99
|
30
|
9
|
| HFE-347mcc3 |
CF3CF2CF2OCH3 |
0.34 |
4.5 (l) |
1600
|
480
|
150
|
| HFE-356pcf3 |
CHF2CF2CH2OCHF2 |
0.39 |
3.2 (n) |
1500
|
430
|
130
|
| HFE-374pc2 |
CHF2CF2OCH2CH3 |
0.25 |
5.0 (n) |
1800
|
540
|
170
|
| HFE-7100 |
C4F9OCH3 |
0.31 |
5.0 (f) |
1300
|
390
|
120
|
| HFE-7200 |
C4F9OC2H5 |
0.30 |
0.77 (g) |
190
|
55
|
17
|
| H-Galden 1040x |
CHF2OCF2OC2F4OCHF2 |
1.37(j) |
6.3 |
5900
|
1800
|
560
|
| HG-10 |
CHF2CHF2OCF2OCHF2 |
0.66 |
12.1 |
7500
|
2700
|
850
|
| HG-01 |
CHFOCFCFCHFOCFCFOCHF2 |
0.87 |
6.2 |
4700
|
1500
|
450
|
 |
|
* The values for CH4 and N2O are adjustment times
including feedbacks of emission on lifetimes (see Chapter
4).
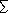 From the formulas
given in Table 6.2, with updated constants
based on the IPCC (l990) expressions. From the formulas
given in Table 6.2, with updated constants
based on the IPCC (l990) expressions.
| Note: For all gases destroyed by reaction with OH, updated
lifetimes include scaling to CH3CCl3 lifetimes,
as well as an estimate of the stratospheric destruction. See references
below for rates along with Chapter 4 and WMO
(l999). |
| (a) Taken from the SAR |
(b) Taken from WMO (l999) |
(c) Taken from WMO (1995) |
(d) DeMore et al. (1997) |
| (e) Good et al. (1998) |
(f) Wallington et al. (1997) |
(g) Christensen et al. (1998) |
(h) Heathfield et al. (1998a) |
| (i) Christidis et al. (1997) |
(j) Gierczak et al. (1996) |
(k) Barry et al. (1997) |
(l) Tokuhashi et al. (1999a) |
| (m) Tokuhashi et al. (1999b) |
(n) Tokuhashi et al. (2000) |
|
|
| |
|
|
|
| † Myhre et al. (l998b) |
†† Jain et al. (2000) |
§ Highwood and Shine (2000) |
& Ko et al. (l999). |
 See Cavalli et al. (l998) and Myhre et al. (l999)
See Cavalli et al. (l998) and Myhre et al. (l999) |
|
The lifetimes and adjustment times used in Tables 6.7
and 6.8 come from Chapter 4
except where noted. For some gases (including several of the fluoroethers),
lifetimes have not been derived from laboratory measurements, but have been
estimated by various other means. For this reason, the lifetimes for these gases,
and hence the GWPs, are considered to be much less reliable, and so these gases
are listed separately in Table 6.8. NF3
is listed in Table 6.8 because, although its photolytic
destruction has been characterised, other loss processes may be significant
but have not yet been characterised (Molina et al., 1995). Note also that some
gases, for example, trifluoromethyl iodide (CF3I) and dimethyl ether
(CH3OCH3) have very short lifetimes (less than a few months);
GWPs for such very short-lived gases may need to be treated with caution, because
the gases are unlikely to be evenly distributed globally, and hence estimates
of, for example, their radiative forcing using global mean conditions may be
subject to error.
Uncertainties in the lifetimes of CFC-11 and CH3CCl3
are thought to be about 10%, while uncertainties in the lifetimes of gases obtained
relative to CFC-11 or CH3CCl3 are somewhat larger (20
to 30%) (SAR; WMO, l999). Uncertainties in the radiative forcing per unit mass
of the majority of the gases considered in Table 6.7 are
approximately ± 10%. The SAR suggested typical uncertainties of ±
35% (relative to the reference gas) for the GWPs, and we retain this uncertainty
estimate for gases listed in Table 6.7. In addition to
uncertainties in the CO2 radiative efficiency per kilogram and in
the response function, AGWPs of CO2 are affected by assumptions concerning
future CO2 abundances as noted above. Furthermore, as the CO2
mixing ratios and climate change, the pulse response function changes as well.
In spite of these dependencies on the choice of future emission scenarios, it
remains likely that the error introduced by these assumptions is smaller than
the uncertainties introduced by our imperfect understanding of the carbon cycle
(see Chapter 3). Finally, although any induced error in
the CO2 AGWPs will certainly affect the non-CO2 GWPs,
it will not affect intercomparisons among non-CO2 GWPs.
![]() From the formulas
given in Table 6.2, with updated constants
based on the IPCC (l990) expressions.
From the formulas
given in Table 6.2, with updated constants
based on the IPCC (l990) expressions.