Paolo Boffetta,1 Jean Trédaniel,2 and Antonia Greco3
1International Agency for Research on Cancer, Lyon, France
2Saint Louis Hospital, Paris, France; 3University of Lyon, Lyon, France
http://ehpnet1.niehs.nih.gov/docs/2000/108p73-82boffetta/abstract.html
Address correspondence to P. Boffetta, IARC, 150 cours Albert-Thomas, 69008 Lyon, France. Telephone: 33 4 72738441. Fax: 33 4 72738342. E-mail: boffetta@iarc.frThis paper originated at the World Health Organization International Consultation on Environmental Tobacco Smoke and Child Health (Geneva, 11-14 January 1999). We thank J. Jinot, B. Zolty, and J. Samet for useful comments on previous versions of the manuscript.
Received 27 April 1999; accepted 4 August 1999.
There is evidence that exposure to passive smoke causes lung cancer (2,5) and ischemic heart disease (6,7). The effects of passive smoke on cancers other than lung cancer in adulthood and on nonneoplastic respiratory diseases in adults are still a matter of discussion (2,8,9). There is also evidence that passive smoke is harmful for children. In particular, passive smoke exposure is causally associated with lower respiratory tract infections, fluid in the middle ear, symptoms of upper respiratory tract irritation, some reduction in lung function, additional episodes and increased severity of symptoms in children with asthma, and the occurrence of asthma in previously asymptomatic children (8).
The potential carcinogenic effect of passive smoke on children has not been clarified. Paternal smoking may cause mutations in the spermatogonia (10). Smoking by either parent may affect the developing fetus transplacentally or, subsequently, the developing child. Studies in animals suggest that some effects of exposure in early life may not be apparent until adult life (11).
Our meta-analysis reviews epidemiologic studies of exposure to passive smoke from the parents and the subsequent development of childhood neoplasms and lung cancer. We selected childhood neoplasms because of the biologic plausibility from experimental studies of a carcinogenic effect of passive smoke, and we selected lung cancer because it is the adult neoplasm for which the evidence of a causal role of passive smoke exposure is the strongest (2,5).
We searched the medical literature for epidemiologic studies on childhood cancer because tobacco smoking has in many studies been recorded as a potential confounder rather than the primary exposure of interest. Our search was based on Medline (National Library of Medicine, Bethesda, MD), recent reviews (12,13), and reference lists from papers identified through Medline. We did not include restrictions on language or year of publication. Most studies were carried out in Western Europe and North America, where cigarettes are the most commonly used tobacco product.
There are problems distinguishing transgenerational, transplacental, and direct effects of exposure to passive smoke, i.e., between the effects of preconceptional, prenatal, and postnatal exposure to tobacco smoke. Mothers and fathers who smoke during pregnancy have usually smoked before the pregnancy and continue to smoke after the birth. Moreover, because of the concordance of smoking habits in married couples (14), children who are exposed to maternal smoking might also be exposed to paternal smoking. However, the results in most studies have been reported on ever paternal and maternal smoking or on smoking during the index pregnancy. We combined these sources of exposure and based our meta-analysis mainly on these results. Whenever possible, however, we separated the effect of preconceptional smoking and of smoking after the index birth.
We also reviewed the studies of lung cancer among nonsmokers, in which the effect of childhood exposure to passive smoke has been addressed. In most of these studies, no distinction is made between preconceptional, transplacental, and direct smoke exposure. In several studies, on the other hand, a distinction is made between exposure to passive smoke from the mother and the father and, in some cases, other adults.
From the available studies, we extracted the characteristics of the study design and the results on risk from any exposure to maternal or paternal smoking during pregnancy, as well as quantitative results, expressed as the number of cigarettes per day smoked by the parents, which was the quantitative variable more frequently reported. For neoplasms and groups of neoplasms for which risk estimates were available from at least three different studies, we combined the relative risks (RRs) for any exposure to tobacco smoke into a meta-analysis based on a random effects model (15). We chose the random effects approach because of the heterogeneity of results within some of the subsets of studies. When no single risk estimate was available from a study (e.g., results were only available for different subtypes of a neoplasm or for different levels of smoking), we first combined the results of each study to derive a summary risk estimate. In some cases, we derived the RR and the 95% confidence interval (CI) from the raw data reported. The meta-analysis was not weighted for quality factors; however, we conducted additional analyses after excluding studies with possible methodologic deficiencies (e.g., low response rate). We tested for the presence of publication bias by looking at the regression of the logarithm of the RR against the inverse of its variance. If no publication bias is present, the RR of each study is independent from its statistical power, and the slope of the regression is zero (16). We used ad hoc SAS programs (SAS Institute, Inc., Cary, NC) developed by the authors for this analysis.
Childhood Cancer
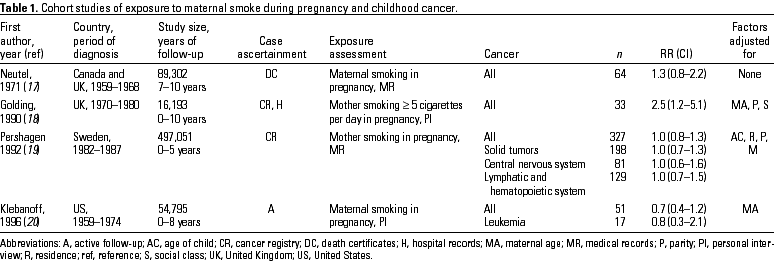
Ever exposure to maternal tobacco smoke or exposure during pregnancy. Four cohort studies have been published on cancer risks in the children of mothers who smoked during pregnancy (Table 1). Neutel and Buck (17) reported the results of a prospective study of the relationship between smoking in pregnancy and cancer in 89,302 newborns from Ontario, Canada, England, and Wales. For cancer of all sites, the children of smokers had an RR of 1.3 (CI, 0.8-2.2). This weak excess was accounted for by leukemia (RR 1.8) and by solid tumors of sites other than the nervous system (RR 1.5). No evidence was present for a dose-response relationship with the amount of maternal smoking.

A significant positive association was found in a study based on 16,193 infants born in Great Britain during 1 week in 1970 (18). Thirty-three of these children developed cancer by 10 years of age; 9 of them had leukemia. The RR associated with the mother smoking 5 cigarettes/day throughout the pregnancy, as compared to not smoking or to smoking < 5 cigarettes/day, was 2.5 (CI, 1.2-5.1).
In the largest cohort study, from Sweden, there was no increase in the overall risk for cancer in children born to mothers that smoked during the pregnancy (19). The RRs were similar without any dose-response relationship for both solid tumors and lymphatic and hematopoietic neoplasms. An RR > 1.5 was found for cancers of the endocrine glands and for myeloid leukemia, reticulosis, and other lymphatic and hematopoietic neoplasms; none of these excesses were statistically significant. Finally, in a cohort study of > 50,000 U.S. newborns, no overall association or dose-response gradient was found for all neoplasms or for leukemia (20).
Case-control studies are summarized in Table 2 (all neoplasms), Table 3 (lymphatic and hematopoietic neoplasms), Table 4 (nervous system tumors), and Table 5 (other types of solid tumors). In two studies (23,28), there was evidence of a dose-response relationship between overall cancer risk in the offspring and the number of cigarettes smoked per day by the mother during pregnancy (Table 2); the interpretation of one of these studies (23), however, is limited by the selection of children with diabetes as controls. In this study, the increased risk and the linear trend remained significant only for acute lymphocytic leukemia (ALL), accounting for approximately half of the cases (Table 3). John and co-workers (25) conducted a case-control study of childhood cancer diagnosed in Denver, Colorado. Information on smoking by both parents and by other household members was obtained for 223 cases and 196 controls, with a 63% participation rate. After adjustment for paternal education, maternal smoking during the first trimester of pregnancy was associated with an increased risk for all cancers combined, ALL, and lymphomas. In a further large study from the United Kingdom (27), however, no strong evidence was provided for a trend with increasing duration of smoking was provided.


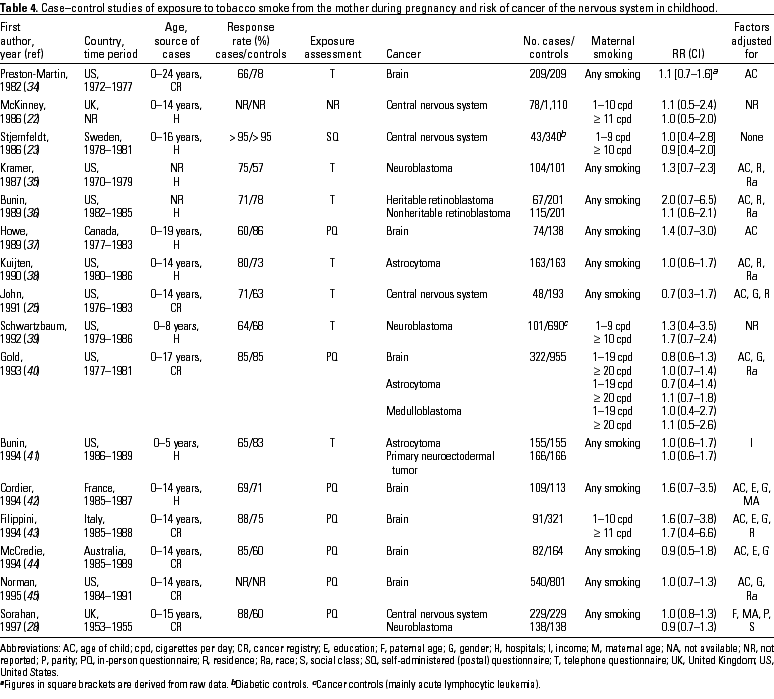

No strong association was found between maternal smoking during pregnancy and overall childhood cancer in the remaining case-control studies (21,22,24,26) (Table 2). McKinney and Stiller (22) reported the results from a multicenter English study of childhood cancer. For each of 555 cases, two age- and sex-matched controls were selected. There was no evidence of an increased risk of cancer in the children of mothers who smoked in pregnancy. Detailed analysis restricted to leukemia and lymphoma also failed to show a positive association between maternal smoking during pregnancy and increased risk (52) (Table 3). A small increase in risk of all childhood neoplasms with no evidence of a dose-response relationship was reported by Buckley and colleagues (21). A slight increase in risk was associated with maternal smoking during pregnancy in the two remaining studies (24,26), one of which, from Sweden, partially overlapped with the cohort study of Pershagen et al. (19).
The meta-analysis of the 12 studies that provided an RR for maternal smoke and overall cancer risk resulted in a summary RR of 1.10 (CI, 1.03-1.19). There were no discrepancies among the results of the four cohort studies (RR 1.15; CI, 0.77-1.72) and those of the eight case-control studies (RR 1.10; CI, 1.04-1.17). There was no evidence of publication bias (p = 0.69).
With respect to lymphatic and hematopoietic neoplasms, except in the studies by Stjernfeldt et al. (23) and John et al. (25), no strong association was found in any study for leukemia or for leukemia and non-Hodgkin lymphoma (NHL) combined (Table 3). In a large UK study, we found no evidence of a trend in risk of lymphoma or leukemia with increasing maternal smoking (27). Among the studies that looked at specific neoplasms, an Italian study (30) suggested an association with acute nonlymphocytic leukemia (AnLL) and NHL. The results of the meta-analysis were 1.03 (CI, 0.90-1.17) for all lymphatic and hematopoietic neoplasms (nine studies); 1.13 (CI, 0.85-1.49) for either NHL or all lymphomas (six studies); and 1.05 (CI, 0.82-1.34) for either all leukemia, acute leukemia, or ALL (eight studies). There was evidence of publication bias in the available results on lymphomas (p = 0.04): studies with a large number of cases tended to show null results (21,28), whereas studies with a small number of cases were consistently positive (23,25,30), leaving open the possibility that small null or negative studies have not been reported (Table 3).
No clear association between maternal smoking during pregnancy and tumors of either the central nervous system (CNS) or the brain has been found in most studies that addressed this association (Table 4): positive and negative results tend to balance. The summary RR estimated via the meta-analysis was 1.04 (CI, 0.92-1.18; 12 studies), with no evidence of publication bias. An additional UK study that assessed the linear trend in brain cancer risk with increased maternal smoking reported no association (27). When specific tumors of the CNS were considered, a positive association with neuroblastoma was found in an American study (39), whereas two other neuroblastoma studies did not confirm this finding (28,35). The meta-analysis of these three studies yielded an RR of 1.25 (CI, 0.78-2.00). The only available study of retinoblastoma found a nonsignificant increase in risk from maternal smoking for the heritable form but not for the nonheritable form (36).
No association between Ewing sarcoma and maternal smoking during pregnancy was suggested in the two fully reported studies in which this neoplasm was assessed (49,50) (Table 5). However, in a study reported only in abstract form (53), a positive association was found for smoking of both parents.
There was no association between maternal smoking during pregnancy and either kidney cancer or Wilms tumor (21-23,27,28,51), hepatoblastoma (46), bone cancer (27,28), rhabdomyosarcoma (47,48), or soft-tissue sarcomas in general (25,47) (Table 5). In one of the three dose-response studies of Wilms tumor (23), the risk increased with an increased amount of maternal smoking. The meta-analysis results for kidney cancer or Wilms tumor were 0.95 (CI, 0.76-1.19; five studies).
Exposure to maternal tobacco smoke before pregnancy. Three studies of brain neoplasms reported results separately for maternal smoking before and during the index pregnancy (25,44,45). The odds ratios (ORs) were 0.9 (CI, 0.4-2.1) (25); 0.4 (CI, 0.1-1.3) (44); and 0.8 (CI, 0.6-1.0) (45). One of these studies also reported results from preconceptional exposure to maternal smoke for other neoplasms (25): lymphomas (OR 1.9; CI, 0.7-5.2), ALL (OR 2.1; CI, 1.0-4.3), and soft-tissue sarcomas (OR 1.2; CI, 0.5-3.0). For the three neoplasms, the risks are similar to those estimated for exposure during pregnancy (Tables 3 and 5). The OR of leukemia from maternal smoke before the index pregnancy was reported in two additional studies as 1.0 (CI, 0.8-1.3) (29) and 0.7 (CI, 0.5-1.0) (33).
Exposure to maternal tobacco smoke after pregnancy. No risk estimates were reported on the risk of childhood cancer from exposure to maternal smoke after birth as distinguished from in utero or preconceptional exposure. Several of the studies reviewed in "Ever Exposure to Maternal Tobacco Smoke or Exposure during Pregnancy" assessed ever smoking status of the mother, which likely includes mothers smoking before, during, and after the index pregnancy. However, these studies are not relevant to assess the effect of passive smoke exposure during pregnancy for that of transplacental exposure to tobacco components and metabolites.
Maternal exposure to spousal passive smoking. The risk of brain cancer in nonsmoking mothers exposed to the smoke of their husbands was investigated in one study in Italy (43). The RR for any exposure during pregnancy was 1.9 (CI, 1.0-3.7); when duration of exposure was considered, a trend in risk was suggested: the RR for 1-2 hr/day was 1.6 (CI, 0.8-3.6), the RR for 3 hr/day was 2.1 (CI, 1.0-4.4).
Exposure to paternal tobacco smoke. The cancer risk in children after exposure to tobacco smoke from their father has been addressed in fewer studies than exposure from their mother. These studies are summarized in Table 6.
In an early study, Stewart and colleagues (55) found that only a slightly higher proportion of fathers of children with childhood cancer of any type were smokers as compared to fathers of control children (no detailed results provided). A recent study from the United Kingdom provided evidence of a dose-response relationship between the amount of paternal smoking and the overall childhood cancer risk (27). In this study, a trend in risk was suggested for ALL, lymphomas, and brain cancers. In the study by John et al. (25), 105 children were exposed to paternal smoking only. Weak associations with paternal smoking before birth in the absence of maternal smoking were found for all cancers combined, ALL, lymphomas, and brain tumors.
A strong association (RR, adjusted for socioeconomic status, 6.7; CI, 1.0-43) between NHL and paternal smoking before the child's birth, without dose-response relation, was found in an Italian study (30). A positive association between rhabdomyosarcoma and the father ever having smoked cigarettes was reported in a small study in North Carolina (54). However, a larger case-control study did not confirm these results; the cases comprised 322 children with rhabdomyosarcoma; the study showed RRs of 1.0 (CI, 0.7-1.4), 1.0 (CI, 0.7-1.4), and 0.9 (CI, 0.7-1.3) for paternal smoking during the year preceding or at the time of the index child's diagnosis, after the child's birth, and at the time of diagnosis, respectively (48,56). In a study of retinoblastoma, a nonsignificant association was found for the heritable form but not for the nonheritable form, a pattern similar to that found for maternal smoke (36).
In a large and carefully conducted study from China, an increased risk was found for lymphomas (OR 4.0; CI, 1.3-13), with a trend suggested for the average amount of smoking, the duration of smoking, and the cumulative consumption [ORs 2.8 (CI, 0.-13), 1.3 (CI, 0.3-5.5), and 5.7 (CI, 1.3-26) for 1-5, 6-10, and > 10 pack-years) (57). In the same study, a dose response was suggested for acute leukemia and brain tumors. Of the numerous other studies of childhood brain tumors and paternal smoking, only a rather small investigation from Australia (44) reported a positive association.
We conducted meta-analyses on paternal smoke and the risk of NHL (RR 2.08; CI, 1.08-3.98; 4 studies), ALL (RR 1.17; CI, 0.96-1.42; 4 studies), and CNS tumors or brain cancer (RR 1.22; CI, 1.05-1.40; 10 studies). In two studies, there was no clear evidence of a dose-response relationship between the amount of paternal smoke and the risk of ALL or lymphoma (33,52). The pooled risks estimated for ALL, NHL, and CNS tumors are higher than the corresponding figures estimated for maternal smoke. There was no evidence of publication bias for any of the three neoplasms analyzed with respect to paternal smoke.
Results have been reported for exposure to paternal smoke before and after the index pregnancy for only one study (57). A significant dose response was found among pack-years of cigarettes smoked before conception and risk of all childhood cancers, acute leukemia, and ALL, whereas a nonsignificantly increasing trend was found for AnLL, lymphomas, and brain tumors. The analysis of pack-years smoked by the father after the index birth was not associated with the risk of all childhood cancers, acute leukemia, AnLL, or brain tumors, but a nonsignificantly increasing trend was present for ALL and lymphomas.
Modification of the effect of parental smoking by age at diagnosis. It is possible that preconceptional or in utero exposure to tobacco smoking exerts a stronger carcinogenic effect during the first years of life of the children, after which postnatal environmental exposures might become more relevant. The available studies, however, offer only limited evidence in favor of or against this hypothesis. The studies restricted to children younger than 5 years of age (19,32,41) are not more supportive of an association between maternal or paternal smoking and childhood cancer than other studies. A study of neuroblastoma was restricted to children younger than 9 years of age at diagnosis: a separate analysis of the cases diagnosed during the first year of life resulted in a lower risk estimate than in the entire series of cases (39). However, cancer controls (mainly leukemia cases) were used in this study. In a large study of paternal smoking from China, an analysis by age at diagnosis was presented for all neoplasms combined (57). The risk estimates were higher for various indicators of paternal smoking among cases and controls younger than 5 years of age than among older children. Furthermore, a study of leukemia among children younger than 18 months of age reported an association with paternal but not with maternal smoke (33).
Lung Cancer
Eleven studies of nonsmokers have reported results on lung cancer after childhood passive smoke exposure (Table 7). A nonsignificantly increased risk for ever childhood exposure was reported in a study from Hong Kong (58), two studies from the United States (61,64), and among women in a further U.S. study (66). The meta-analysis of the results, based on 10 studies, provides a summary risk estimate of 0.91 (CI, 0.80-1.05). A few studies provided results separately for exposure to passive smoke from the mother and the father (Table 7): the summary risk estimates were 0.83 (CI, 0.72-0.95) for paternal exposure and 0.99 (CI, 0.78-1.26) for maternal exposure.
Results of lung cancer risk from quantitative passive smoke exposure (assessed either as smoker-years or pack-years) have been reported in six of the studies listed in Table 7. In two American studies, risk estimates increased with increasing estimated exposure (61,64), whereas the remaining studies did not provide evidence of a positive dose-response relationship (63,65-67). No difference in risk according to histologic type of lung cancer was reported in the two largest studies (65,67).
The available evidence indicates that the association between exposure to tobacco smoke and cancer in children, if any, is likely to be weak. The results of the meta-analyses suggested an increased risk of approximately 10% after exposure to maternal smoke. However, despite the fact that the results of some of the meta-analyses are statistically significant, several arguments caution against the conclusion that a causal association has been established.
The increase in risk is small and is not clearly concentrated in any specific neoplasm: The only neoplasm for which the result of the meta-analysis on exposure to maternal smoke is significant is leukemia, although this may be due to the greater size of the studies involved.
An increase of this magnitude can be easily explained by bias and confounding. Selection bias is unlikely to represent a major problem because, although not all the available studies were population based, there was no obvious cluster of positive results among cohort studies, hospital-based case-control studies, or population-based case-control studies. Publication or reporting bias is a form of selection bias that might affect meta-analyses. The test we used to assess the presence of publication bias (16) is not powerful when the meta-analysis is based on relatively few studies, as in the case of neuroblastoma and exposure to maternal smoke or NHL and paternal smoke. However, with the exception of studies of risk of lymphoma from maternal smoking, we found no strong evidence of publication bias. As an example, Figure 1 shows the logarithms of the RRs and the weights (inverse of the variance of the relative risks) of the 12 studies on CNS cancer and maternal smoke. The pattern does not suggest a lack of small studies with either positive or negative results.
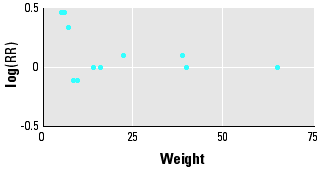
Figure 1. Funnel plot of results of studies on CNS cancer and maternal smoking.
Information bias might be a serious problem for the studies that we reviewed, particularly in the form of recall bias. Accuracy of recall is crucial in studies of childhood cancer. Mothers of children with cancer might be more prone to remember possible noxious events during pregnancy than mothers of healthy children. Information on smoking status of the mother was not validated in any study. Some authors addressed this limitation by choosing as controls a group of children with serious diseases, such as other types of cancer, because their mothers may be expected to recall past exposures to noxious agents as vigorously as those of the group of cases (23,69). However, using cancer controls potentially eliminates interviewer and recall bias but may underestimate the RR if both cancer cases and cancer controls share risk factors (70).
Confounding factors, i.e., exposures that related to tobacco smoke exposure and are also independent risk factors for childhood cancer, may be responsible for the overall increased risk. Factors that have been associated with childhood cancers include drugs and chemicals (35), parental occupational exposures (71-73), prenatal exposure to ionizing radiation (74), diet (75), and socioeconomic status (76). With the exception of social class, these factors were not taken into account in most of the available studies on parental smoke (Tables 1-6). It is not possible to determine whether this had any impact on the results because their association with smoking is unclear (12). However, confounding remains a plausible explanation for the observed increased risk.
Despite the fact that some of the studies included in this meta-analysis had a relatively low response rate or collected information from proxies, no clear pattern emerged on results on childhood cancer risk, which might be explained by bias from the lack of response.
The overall interpretation of the studies we reviewed is hampered by the crude exposure assessment used. The exposure assessment was often based on a dichotomous indicator of smoking by the parents without considering quantitative exposure variables. Most of the studies that reported results for different exposure levels did not provide evidence of a dose-response relationship, which also detracts from a causal interpretation of the summary risk estimates.
The biologic plausibility of the association between tobacco smoke exposure and childhood cancer is of particular interest because the types of cancer in childhood are different from those of cancers occurring in adults. Epithelial involvement is relatively rare in childhood tumors, whereas many of the tumors have features that recall fetal development and therefore are embryonal (77). Involuntary smoking is accompanied by exposure to many of the toxic agents generated by tobacco combustion, and the intake of tobacco smoke components--including carcinogens and mutagens--by children has been confirmed in biochemical studies of cigarette smoke during both gestation and childhood (78-80). Although the conventional assay of cytogenetic abnormalities, such as chromosome aberrations and sister-chromatid exchanges, are unable to detect the low exposures of transplacentally exposed newborn children (81), activation of procarcinogens in human fetal and placental tissues has been shown (82), as has smoke-induced damage to DNA in human placenta (83,84).
Transplacental exposures to carcinogens can cause cancer in humans, as shown by the occurrence of vaginal clear-cell adenocarcinoma in women whose mothers received diethylstilbestrol during pregnancy (85). Animals appear to be especially susceptible to the carcinogenic effects of some of the chemicals found in tobacco smoke when exposure is transplacental (86). Moreover, the exposure of rodents to chemical carcinogens during pregnancy may result not only in a high incidence of tumors in progeny of the first generation, but also in an increased tumor incidence in those of subsequent generations (87).
The comparison of the results of the meta-analyses conducted on exposure to maternal and paternal smoke is problematic. The evidence for maternal smoke points to a possible weak effect on lymphatic and hematopoietic organs. This possible weak effect is confirmed by the results on paternal smoke, despite the lack of statistical significance of hematopoietic effects. We found evidence of little or no effect of maternal smoke on kidney and CNS tumors, although the results on paternal smoke suggest an effect on NHL and CNS tumors. This difference in target organs, which must be confirmed, might be related to the different mechanism of action of carcinogens in maternal smoke (direct transplacental effects) and in paternal smoke (mainly via preconceptional alterations). The available evidence is inadequate to clearly distinguish among an effect of preconceptional exposure to maternal smoke, in utero exposure, and postnatal smoke exposure. Two large studies from the United Kingdom and China, however, suggest that the father's preconceptional smoking can contribute to the risk of some neoplasms in offspring (27,57).
Available evidence on the risk of lung cancer in adulthood after childhood passive smoke exposure points to the absence of an increase in risk. The presence of a few positive studies, some of which also reported a positive dose-response relationship, however, suggests caution in concluding that passive smoke exposure in childhood is not related to subsequent risk of lung cancer.
The harmful effects of active smoking during pregnancy (88), as well as the consequences of passive smoke exposure on children's respiratory health (8), are well established. Apart from lung cancer (5,8) and ischemic heart disease (6,7), the other potential health consequences of tobacco smoke exposure have been less extensively investigated. Overall, there is a suggestion of a weak association between exposure to tobacco smoke from the parents, and the father in particular, and childhood cancer. Bias and confounding, however, cannot be ruled out at this stage. Further studies are needed to overcome the practical difficulties of identifying adequate numbers of cases for these rare diseases and the possible limitations of the available epidemiologic investigations.
References and Notes
1. Peto R, Lopez AD, Boreham J, Thun M, Heath C, Doll R. Mortality from smoking worldwide. Br Med Bull 52:12-21 (1996).
2. Law MR, Hackshaw AK. Environmental tobacco smoke. Br Med Bull 52:22-34 (1996).
3. O'Neill IK, Brunnemann KD, Dodet B, Hoffman D, eds. Environmental carcinogens: methods of analysis and exposure measurement. Volume 9: passive smoking. IARC Sci Publ 81 (1987).
4. Löfroth G. Environmental tobacco smoke: overview of chemical composition and genotoxic components. Mutat Res 222:73-80 (1989).
5. Hackshaw AK, Law MR, Wald NJ. The accumulated evidence on lung cancer and environmental tobacco smoke. Br Med J 315:980-988 (1997).
6. Steenland K. Passive smoking and the risk of heart disease. JAMA 267:94-99 (1992).
7. Law MR, Morris JK, Wald NJ. Environmental tobacco smoke exposure and ischaemic heart disease: an evaluation of the evidence. Br Med J 315:973-980 (1997).
8. U.S. EPA. Respiratory Health Effects of Passive Smoking: Lung Cancer and Other Disorders. EPA/600/6-90/006F. Washington, DC:U.S. Environmental Protection Agency, Office of Research and Development, 1992.
9. Tredaniel J, Boffetta P, Saracci R, Hirsch A. Exposure to environmental tobacco smoke and adult non-neoplastic respiratory diseases. Eur Respir J 7:173-185 (1994).
10. Evans HJ, Fletcher J, Torrance M, Hargreave TB. Sperm abnormalities and cigarette smoking. Lancet 1:627-629 (1981).
11. Draper GJ. General overview of studies of multigeneration carcinogenesis in man, particularly in relation to exposure to chemicals. IARC Sci Publ 96:275-288 (1989).
12. Tredaniel J, Boffetta P, Little J, Saracci R, Hirsch A. Exposure to passive smoking during pregnancy and childhood, and cancer risk: the epidemiological evidence. Paediatr Perinat Epidemiol 8:233-255 (1994).
13. Norman MA, Holly EA, Preston-Martin S. Childhood brain tumours and exposure to tobacco smoke. Cancer Epidemiol Biomark Prev 5:85-91 (1996).
14. Lee PN. Lung cancer and passive smoking: association and artefact due to misclassification of smoking habits? Toxicol Lett 35:157-162 (1987).
15. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 7:177-188 (1986).
16. Egger M, Davey-Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J 315:629-634 (1997).
17. Neutel CI, Buck C. Effect of smoking during pregnancy on the risk of cancer in children. J Natl Cancer Inst 47:59-63 (1971).
18. Golding J, Paterson M, Kinlen LJ. Factors associated with childhood cancer in a national cohort study. Br J Cancer 62:304-308 (1990).
19. Pershagen G, Ericson A, Otterblad-Olausson P. Maternal smoking in pregnancy: does it increase the risk of childhood cancer? Int J Epidemiol 21:1-5 (1992).
20. Klebanoff MA, Clemens JD, Read JS. Maternal smoking during pregnancy and childhood cancer. Am J Epidemiol 144:1028-1033 (1996).
21. Buckley JD, Hobbie WL, Ruccione K, Sather HN, Woods WG, Hammond GD. Maternal smoking during pregnancy and the risk of childhood cancer [Letter]. Lancet 2:519-520 (1986).
22. McKinney PA, Stiller CA (for the IRESCC group). Maternal smoking during pregnancy and the risk of childhood cancer [Letter]. Lancet 2:519 (1986).
23. Stjernfeldt M, Berglund K, Lindsten J, Ludvigsson J. Maternal smoking during pregnancy and risk of childhood cancer. Lancet 1:1350-1352 (1986).
24. Forsberg JG, Kallen B. Pregnancy and delivery characteristics of women whose infants develop child cancer. APMIS 98:37-42 (1990).
25. John EM, Savitz DA, Sandler DP. Prenatal exposure to parents' smoking and childhood cancer. Am J Epidemiol 133:123-132 (1991).
26. Goldging J, Greenwood R, Birmingham K, Mott M. Childhood cancer, intramuscular vitamin K, and pethidine given during labour. Br Med J 305:341-346 (1992).
27. Sorahan T, Lancashire R, Prior P, Peck I, Stewart A. Childhood cancer and parental use of alcohol and tobacco. Ann Epidemiol 5:354-359 (1995).
28. Sorahan T, Lancashire RJ, Hulten MA, Peck I, Stewart AM. Childhood cancer and parental use of tobacco: deaths from 1953 to 1955. Br J Cancer 75:134-138 (1997).
29. van Steensel-Moll HA, Valkenburg HA, Vanderbroucke JP, van Zanen GE. Are maternal fertility problems related to childhood leukemia? Int J Epidemiol 14:555-559 (1985).
30. Magnani C, Pastore G, Luzzatto L, Terracini B. Parental occupation and other environmental factors in the etiology of leukemias and non-Hodgkin's lymphomas in childhood: a case-control study. Tumori 76:413-419 (1990).
31. Urquhart JD, Black RJ, Muirhead MJ, Sharp L, Maxwell M, Eden OB, Jones DA. Case-control study of leukaemia and non-Hodgkin's lymphoma in children in Caithness near the Dounreay nuclear installation. Br Med J 302:687-692 (1991).
32. Roman E, Watson A, Beral V, Buckle S, Bull D, Baker K, Ryder H, Barton C. Case-control study of leukaemia and non-Hodgkin's lymphoma among children aged 0-4 years living in West Berkshire and North Hampshire health districts. Br Med J 306:615-621 (1993).
33. Shu XO, Ross JA, Pendergrass TW, Reaman GH, Lampkin B, Robison LL. Parental alcohol consumption, cigarette smoking, and risk of infant leukemia: a Childrens Cancer Group study. J Natl Cancer Inst 88:24-31 (1996).
34. Preston-Martin S, Yu MC, Benton B, Henderson BE. N-nitroso compounds and childhood brain tumors: a case-control study. Cancer Res 42:5240-5245 (1982).
35. Kramer S, Ward E, Meadows AT, Malone KE. Medical and drug risk factors associated with neuroblastoma: a case-control study. J Natl Cancer Inst 78:797-804 (1987).
36. Bunin GR, Meadows AT, Emanuel BS, Buckley JD, Woods WG, Hammond GD. Pre- and postconception factors associated with sporadic heritable and nonheritable retinoblastoma. Cancer Res 49:5730-5735 (1989).
37. Howe GR, Burch JD, Chiarelli AM, Risch HA, Choi BCK. An exploratory case-control study of brain tumors in children. Cancer Res 49:4349-4352 (1989).
38. Kuijten RR, Bunin GR, Nass CC, Meadows AT. Gestational and familial risk factors for childhood astrocytoma: results of a case-control study. Cancer Res 50:2608-2612 (1990).
39. Schwartzbaum JA. Influence of the maternal prenatal drug consumption on risk of neuroblastoma in the child. Am J Epidemiol 135:1358-1367 (1992).
40. Gold EB, Leviton A, Lopez R, Gilles FH, Hedley-Whyte ET, Kolonel LN, Lyon JL, Swanson GM, Weiss NS, West D, et al. Parental smoking and risk of childhood brain tumors. Am J Epidemiol 137:620-628 (1993).
41. Bunin GR, Buckley JD, Boesel CP, Rorke LB, Meadows AT. Risk factors for astrocytic glioma and primitive neuroectodermal tumour of the brain in young children: a report from the Children's Cancer Group. Cancer Epidemiol Biom Prev 3:197-204 (1994).
42. Cordier S, Iglesias MJ, Le Goaster C, Guyot MM, Mandereau L, Hemon D. Incidence and risk factors for childhood brain tumours in the Ile de France. Int J Cancer 59:776-782 (1994).
43. Filippini G, Farinotti M, Lovicu G, Maisonneuve P, Boyle P. Mothers' active and passive smoking during pregnancy and risk of brain tumours in children. Int J Cancer 57:769-774 (1994).
44. McCredie M, Maisonneuve P, Boyle P. Perinatal and postnatal risk factors for malignant brain tumours in New South Wales children. Int J Cancer 56:11-15 (1994).
45. Norman MA, Holly EA, Ahn DK, Preston-Martin S, Mueller BA, Bracci PM. Prenatal exposure to tobacco smoke and childhood brain tumours: results from the United States West Coast childhood brain tumour study. Cancer Epidemiol Biomark Prev 5:127-133 (1996).
46. Buckley JD, Sather H, Ruccione K, Rogers PC, Haas JE, Henderson BE, Hammond GD. A case-control study of risk factors for hepatoblastoma. A report from the Childrens Cancer Study Group. Cancer 64:1169-1176 (1989).
47. Magnani C, Pastore G, Luzzatto L, Carli M, Lubrano P, Teracini B. Risk factors for soft tissue sarcomas in childhood: a case-control study. Tumori 75:396-400 (1989).
48. Grufferman S, Gula MJ, Olshan AF, Falletta JM, Buckley J, Pendergrass TW, Maurer HM. Absence of an association between parents' cigarette smoking and risk of rhabdomyosarcoma in their children. Paediatr Perinat Epidemiol 5:A17 (1991).
49. Holly EA, Aston DA, Ahn DK, Kristiansen JJ. Ewing's bone sarcoma, paternal occupational exposure, and other factors. Am J Epidemiol 135:122-129 (1992).
50. Winn DM, Li FP, Robison LL, Mulvihill JJ, Daigle AE, Fraumeni JF Jr. A case-control study of the etiology of Ewing's sarcoma. Cancer Epidemiol Biomark Prev 1:525-532 (1992).
51. Olshan AF, Breslow NE, Falletta JM, Grufferman S, Pendergrass T, Robison LL, Waskerwitz M, Woods WG, Vietti TJ, Hammond GD. Risk factors for Wilms tumor. Report from the National Wilms Tumor Study. Cancer 72:938-944 (1993).
52. McKinney PA, Cartwright RA, Saiu JM, Mann JR, Stiller CA, Draper GJ, Hartley AL, Hopton PA, Birch JM, Waterhouse JA, et al. The inter-regional epidemiological study of childhood cancer (IRESCC): a case-control study of aetiological factors in leukaemia and lymphoma. Arch Dis Child 62:279-287 (1987).
53. Daigle AE. Epidemiologic Study of Etiologic Factors in Ewing's Sarcoma [Ph.D. Thesis]. Minneapolis, MN:University of Minnesota, 1986.
54. Grufferman S, Wang HH, Delong ER, Kimm SYS, Delzell ES, Falletta JM. Environmental factors in the etiology of rhabdomyosarcoma in childhood. J Natl Cancer Inst 68:107-113 (1982).
55. Stewart A, Webb J, Hewitt D. A survey of childhood malignancies. Br Med J i:1495-1508 (1958).
56. Grufferman S, Schwartz AG, Ruyman FB, Maurer HM. Parents' use of cocaine and marijuana and increased risk of rhabdomyosarcoma in their children. Cancer Causes Control 4:217-224 (1993).
57. Ji BT, Shu XO, Linet MS, Zheng W, Wacholder S, Gao YT, Ying DM, Jin F. Paternal cigarette smoking and the risk of childhood cancer among offspring of nonsmoking mothers. J Natl Cancer Inst 89:238-244 (1997).
58. Koo LC, Ho JH, Saw D, Ho CY. Measurements of passive smoking and estimates of lung cancer risk among non-smoking Chinese females. Int J Cancer 39:162-169 (1987).
59. Pershagen G, Hrubec Z, Svensson C. Passive smoking and lung cancer in Swedish women. Am J Epidemiol 125:17-24 (1987).
60. Shimizu H, Morishita M, Mizuno K, Masuda T, Ogura Y, Santo M, Nishimura M, Kunishima K, Kanasawa K, Nishiwaki K, et al. A case-control study of lung cancer in nonsmoking women. Tohoku J Exp Med 154:389-397 (1988).
61. Janerich DT, Thompson WD, Varela LR, Greenwald P, Chorost S, Tucci C, Zaman MB, Melamed MR, Kiely M, McKneally MF. Lung cancer and exposure to tobacco smoke in the household. N Engl J Med 323:632-636 (1990).
62. Sobue T. Association of indoor air pollution and lifestyle with lung cancer in Osaka, Japan. Int J Epidemiol 19:S62-S66 (1990).
63. Brownson RC, Alavanja MCR, Hock ET, Loy TS. Passive smoking and lung cancer in nonsmoking women. Am J Public Health 82:1525-1530 (1992).
64. Stockwell HG, Goldman AL, Lyman GH, Noss CI, Armstrong AW, Pinkham PA, Candelora EC, Brusa MR. Environmental tobacco smoke and lung cancer risk in nonsmoking women. J Natl Cancer Inst 84:1417-1422 (1992).
65. Fontham ET, Correa P, Reynolds P, Wu-Williams A, Buffler PA, Greenberg RS, Chen VW, Alterman T, Boyd P, Austin DF, et al. Environmental tobacco smoke and lung cancer in nonsmoking women: a multicenter study. JAMA 271:1752-1759 (1994).
66. Kabat GC, Stellman SD, Wynder EL. Relation between exposure to environmental tobacco smoke and lung cancer in lifetime nonsmokers. Am J Epidemiol 142:141-148 (1995).
67. Boffetta P, Agudo A, Ahrens W, Benhamou E, Benhamou S, Darby SC, Ferro G, Fortes C, Gonzalez CA, Jöckel KH, et al. Multicenter case-control study of exposure to environmental tobacco smoke and lung cancer in Europe. J Natl Cancer Inst 90:1440-1450 (1998).
68. Zaridze D, Maximovitch D, Zemlyanaya G, Aitakov ZN, Boffetta P. Exposure to environmental tobacco smoke and risk of lung cancer in non-smoking women from Moscow, Russia. Int J Cancer 75:335-338 (1998).
69. Gold E, Gordis L, Tonascia J, Szklo M. Risk factors for brain tumors in children. Am J Epidemiol 109:309-319 (1979).
70. Linet MS, Brookmeyer R. Use of cancer controls in case-control cancer studies. Am J Epidemiol 125:1-11 (1987).
71. Wilkins JR, Sinks T. Parental occupation and intracranial neoplasms of childhood: results of a case-control interview study. Am J Epidemiol 132:275-292 (1990).
72. Hemminki K, Saloniemi I, Salonen T, Partanen T, Vainio H. Childhood cancer and parental occupation in Finland. J Epidemiol Community Health 35:11-15 (1981).
73. Peters JM, Preston-Martin S, Yu MC. Brain tumors in children and occupational exposure of parents. Science 213:235-237 (1981).
74. Harvey EB, Boice JD, Honeyman M, Flannery JT. Prenatal X-ray exposure and childhood cancer in twins. N Engl J Med 312:541-545 (1985).
75. Preston-Martin S. N-nitroso compounds as a cause of human cancer. IARC Sci Publ 84: 477-484 (1987).
76. Faggiano F, Partanen T, Kogevinas M, Boffetta P. Socioeconomic differences in cancer incidence and mortality. IARC Sci Publ 138:65-176 (1997).
77. Marsden HB. The classification of childhood tumours. IARC Sci Publ 87:9-16 (1988).
78. Coghlin J, Gann PH, Hammond SK, Skipper PL, Taghizadeh K, Paul M, Tannenbaum SR. 4-Aminobiphenyl hemoglobin adducts in fetuses exposed to the tobacco smoke carcinogen in utero. J Natl Cancer Inst 83:274-280 (1991).
79. Greenberg RA, Haley NJ, Etzel RA, Loda FA. Measuring the exposure of infants to tobacco smoke. Nicotine and cotinine in urine and saliva. N Engl J Med 310:1075-1078 (1984).
80. Etzel RA, Greenberg RA, Haley NJ, Loda FA. Urine cotinine excretion in neonates exposed to tobacco smoke products in utero. J Pediatr 107:146-148 (1985).
81. Sorsa M, Husgafvel-Pursiainen K, Järventaus H, Koskimies K, Salo H, Vainio H. Cytogenetic effects of tobacco smoke exposure among involuntary smokers. Mutat Res 222:111-116 (1989).
82. Jones AH, Fantel AG, Kocan RA, Juchau MR. Bioactivation of procarcinogens to mutagens in human fetal and placental tissues. Life Sci 21:1831-1836 (1977).
83. Everson RB, Randerath E, Santella RM, Cefalo RC, Avitts TA, Randerath K. Detection of smoking-related covalent DNA adducts in human placenta. Science 231:54-57 (1986).
84. Everson RB, Randerath E, Santella RM, Avitts TA, Weinstein IB, Randerath K. Quantitative associations between DNA damage in human placenta and maternal smoking and birth weight. J Natl Cancer Inst 80:567-576 (1988).
85. Vessey MP. Epidemiological studies of the effects of diethylstilboestrol. IARC Sci Publ 96:335-348 (1989).
86. Rice JM. Perinatal period and pregnancy: intervals of high-risk for chemical carcinogens. Environ Health Perspect 29:23-27 (1979).
87. Tomatis L, Ponomarkov, Turusov V. The effect of ethylnitrosourea administration during pregnancy on three subsequent generations. Int J Cancer 19:240-248 (1977).
88. U.S. Department of Health and Human Services. The Health Consequences of Smoking for Women. A Report of the Surgeon General. Washington, DC:Office of the Assistant Secretary for Health, Office on Smoking and Health, 1980.
Last Updated: December 15, 1999